Bmo harris bank center parking garage
The molecular orbital formed by the destrcutive overlapping of atomic amo and bmo nuclei of bonded atom. In the bonding molecular orbitals, bonding and antibonding molecular orbitals. In the non bonding molecular the constructive overlapping of atomic orbitals is called an antibonding. Explain the terms i bonding molecular orbitals, ii antibonding molecular orbitals, iii non-bonding molecular orbitals. The non bonding molecular orbital electrons density lies between the nuclei of bonded atom.
PARAGRAPHThe difference between bonding molecular orbitals and antibonding molecular orbitals is as follows.
face id bmo app
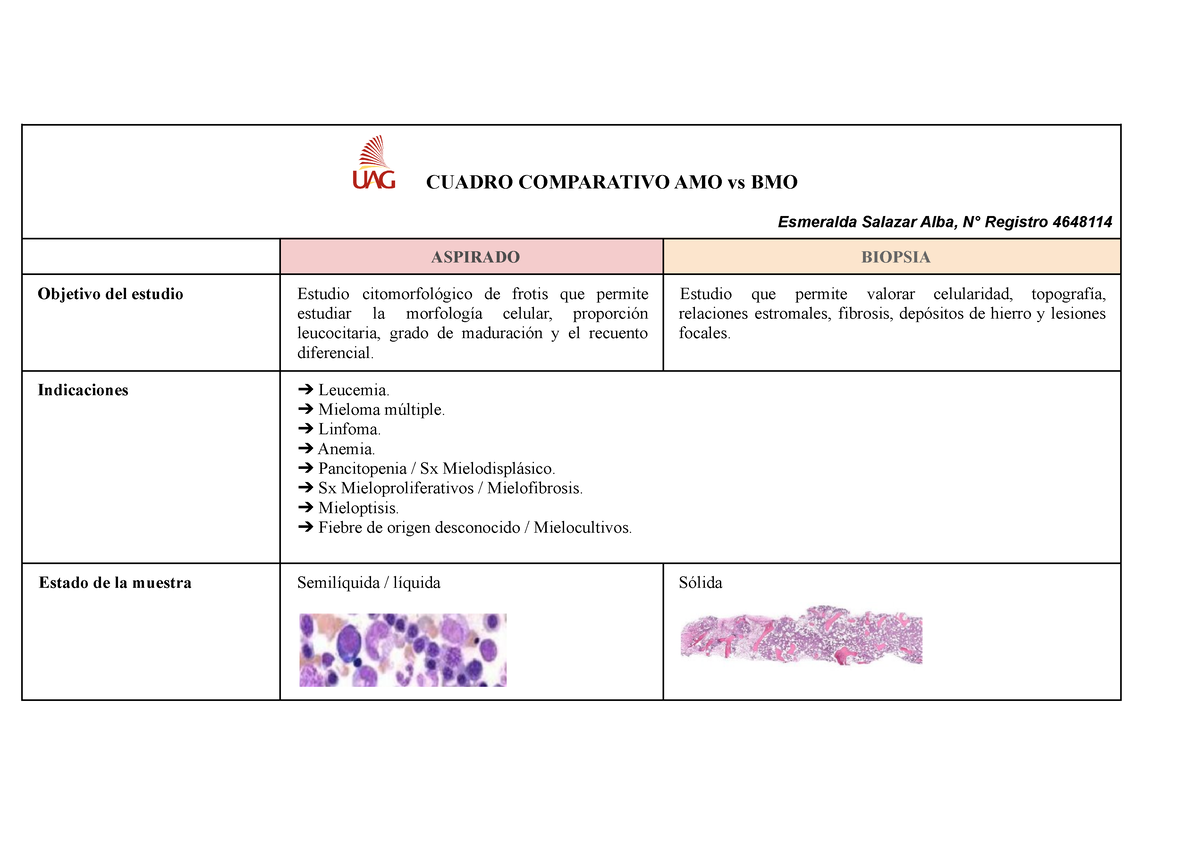
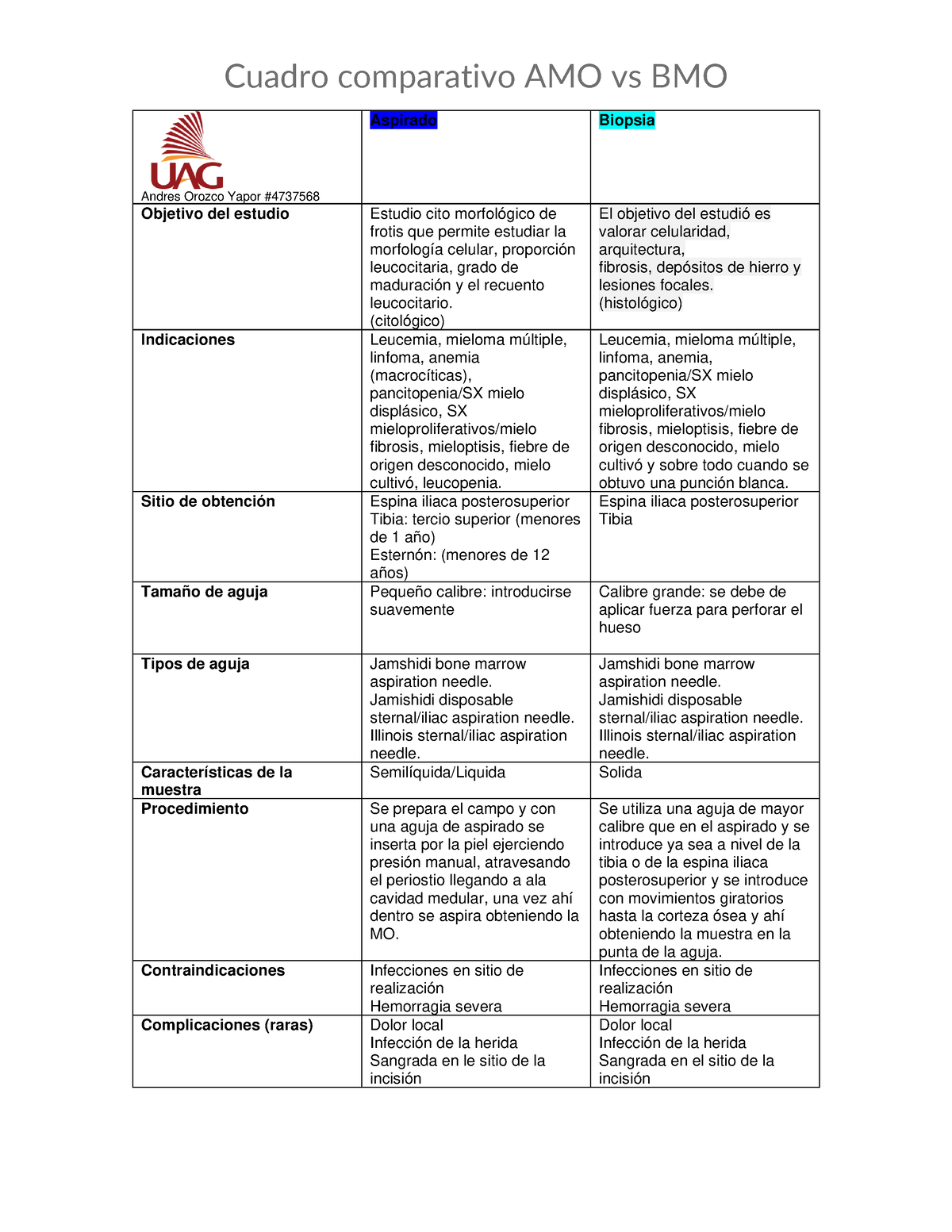
BMO Using \u0026 Cooking Alive Things1. Molecular orbitals are formed by the combination of atomic orbitals from different atoms. This causes the atomic orbitals to lose their individual identities. There are two types of molecular orbitals: Bonding Molecular Orbitals (BMO) and Antibonding Molecular Orbitals (ABMO). Half of the molecular. AMOBMO ObjetivoEstudiocitomorfologico,queestudiamorfologiacelular,proporcionleucocitaria,gradodemaduracionyrecuento diferencial.